September 3, 2024
7 Skin Cancer Cells Misconceptions Exposed Fred Hutchinson Cancer Cells Center
Afamelanotide Wikipedia
The Centers for Disease Control and Prevention advises applying a thick layer of a wide range sunscreen (that is, one that protects versus both UVA and UVB light) of at least SPF 15 prior to going outside, even on cloudy or trendy days. Sunscreen should be reapplied after 2 hours in the sunlight or after swimming, sweating or using a towel. " There are some individuals that are vulnerable to addictive habits who are vulnerable to suntanning for the same reasons," Cranmer stated. Afamelanotide is an injectable subcutaneous implant made use of to mitigate phototoxicity second to erythropoietic protoporphyria (EPP).
Metrology Of Npy Mrna
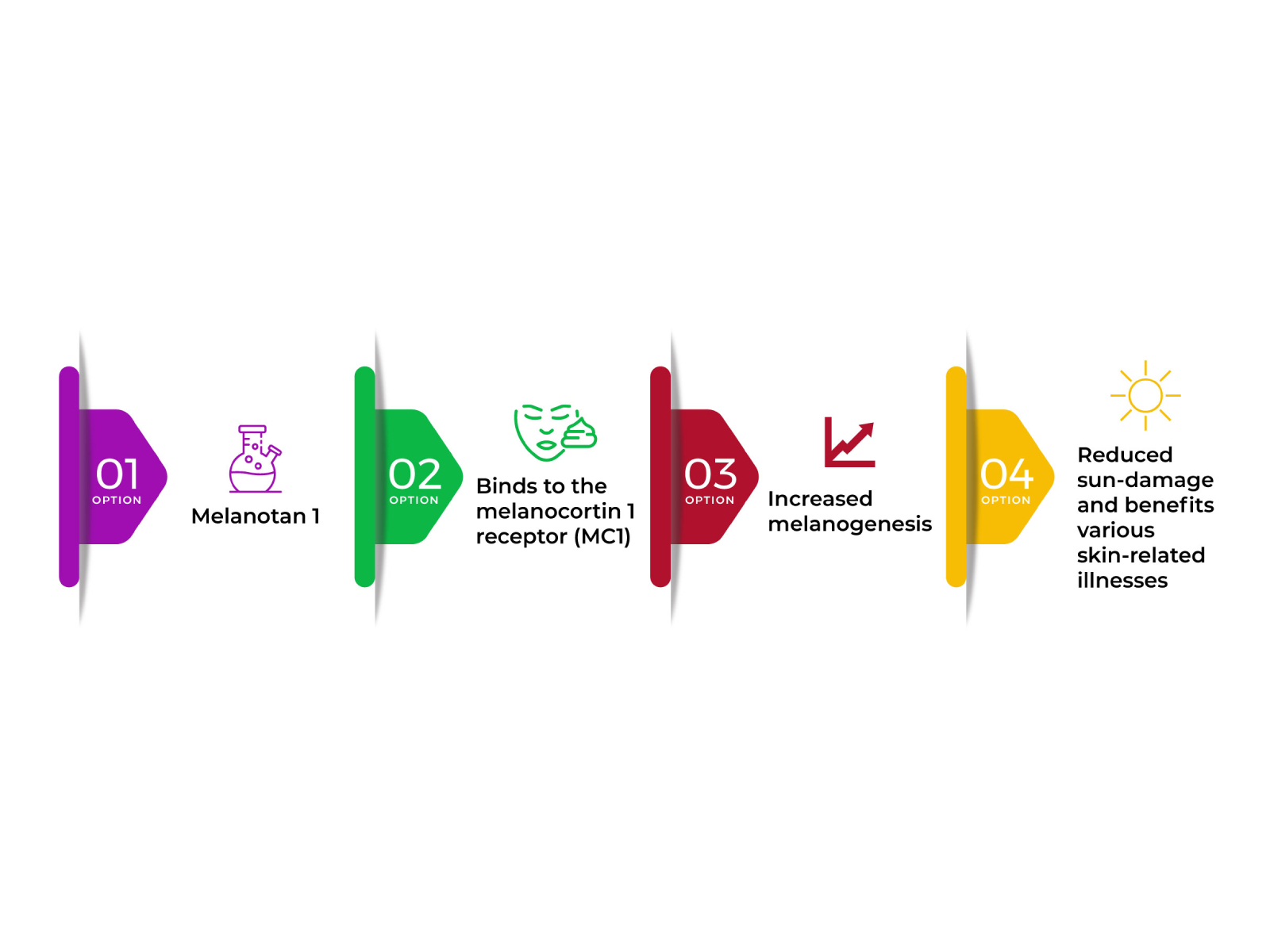
Since nursing varies substantially from adult feeding behavior, a number of previous researches have utilized models of adult-like independent ingestion to study the ontogeny of food intake controls in pups (30-- 33). These research studies have shown that, prior to P6, food intake is mainly hindered by gastric fill (34, 35), and, by P9, independent intake can be prevented by nutritive signals (1, 36). In comparison, nutritive signals do not appear to hinder suckling up until a minimum of P14 (35, 37). We, nevertheless, observed MTII-mediated inhibition of milk consumption in suckling dogs at all ages studied, from P6 to P16. This restraint therefore does not appear to mirror the developmental progression of inhibitory ingestive controls yet instead most likely shows activation of main melanocortin receptors that are already present at birth. Importantly, these researches show that, not just does MTII hinder strong food consumption in grown-up rats, yet it can inhibit suckling-mediated milk consumption as very early as P6, a time when food intake is mostly moderated by gastric fill (1 ).
Just How Are Anabolic Steroids Checked In Professional Athletes?
It consists of 16 mg of afamelanotide and is implanted under the skin, usually around the hip. It ought to be put by an expert doctor every 2 months, prior to and throughout raised sunshine direct exposure (eg, summer). It is suggest to have 3 implants each year, with an optimum of four each year. Since this writing, available information concerning Melanotan II appears to be from the firm itself, consisting of the article on Melanotan II on Wikipedia (the online, open-source encyclopedia resource). Melanocorp, Inc.'s official website has for the time being removed its website advertising and marketing Melanotan II, though FDA spokespeople think various other websites could still sell the potentially dangerous product. Wikipedia editors intend to quickly eliminate the deceptive details (taken into consideration marketing) concerning this skin sun tanning item.

- This inhibition consequently does not appear to reflect the developing development of inhibitory ingestive controls yet instead likely mirrors activation of central melanocortin receptors that are already existing at birth.
- Melanocorp, Inc.'s main website has for the time being removed its web site advertising and marketing Melanotan II, though FDA spokespeople think other sites may still market the potentially hazardous product.
- They ultimately created an additional analog, Ac-Nle-cyclo [Asp-His-D-Phe-Arg-Trp-Lys] -NH2), which they called "Melanotan II".
- Although the major orexigenic neurocircuitry, i.e. the ARH NPY/AgRP neuronal forecasts, are not established in the very early postnatal period, hypothalamic NPY material is bountiful throughout this moment.
When using an uncontrolled product, you run the risk of breathing in and taking in excessive of the medication and presenting impurities and impurities right into your body. So, also if you want to take a chance on the prospective health and wellness risks included with using melanotan, you might wind up with an item which contains pollutants or hazardous ingredients. The hashtags #BarbieFeet and #BarbieFeetChallenge have amassed a consolidated total of over 80 million views on the platform, and search rate of interest for "Barbie Foot Challenge" enhanced 2,950% throughout one week, Foot, Ankle Joint & Leg Capillary Center reported. A reporter from the outlet got the products from 3 sellers on TikTok-- @Supasuppsuk, @Melanin_MagicTanning and @tropicbarbiex-- every one of which arrived in sparkly pink product packaging, with one including complimentary sweet, also. The neuroanatomical pathways moderating melanocortin effects on BAT thermogenesis are believed to entail PVH nerve cells that reveal melanocortin receptors. Intra-PVH MTII administration both raises oxygen consumption and inhibits food intake (41 ). We also demonstrated previously an increase in UCP1 mRNA Click for source degrees in feedback to intra-DMH MTII administration in lactating rats (12 ). Furthermore, there appears to be an independent path in the caudal brainstem, as shown by raised UCP1 mRNA in BAT after fourth ventricle MTII administration in chronic decerebrate rats (39 ). Due to the fact that we observed MTII-induced c-Fos activation in both the hypothalamus and the brainstem in rat dogs, the UCP1 activation and impacts on food consumption might have been moderated by either of these paths. Although our research studies demonstrate that rat puppies have the capability for anorexigenic impacts, orexigenic drive is anticipated to control during development to sustain rapid development.
Social Links