September 5, 2024
Tesofensine Peptide In Midlothian, Va
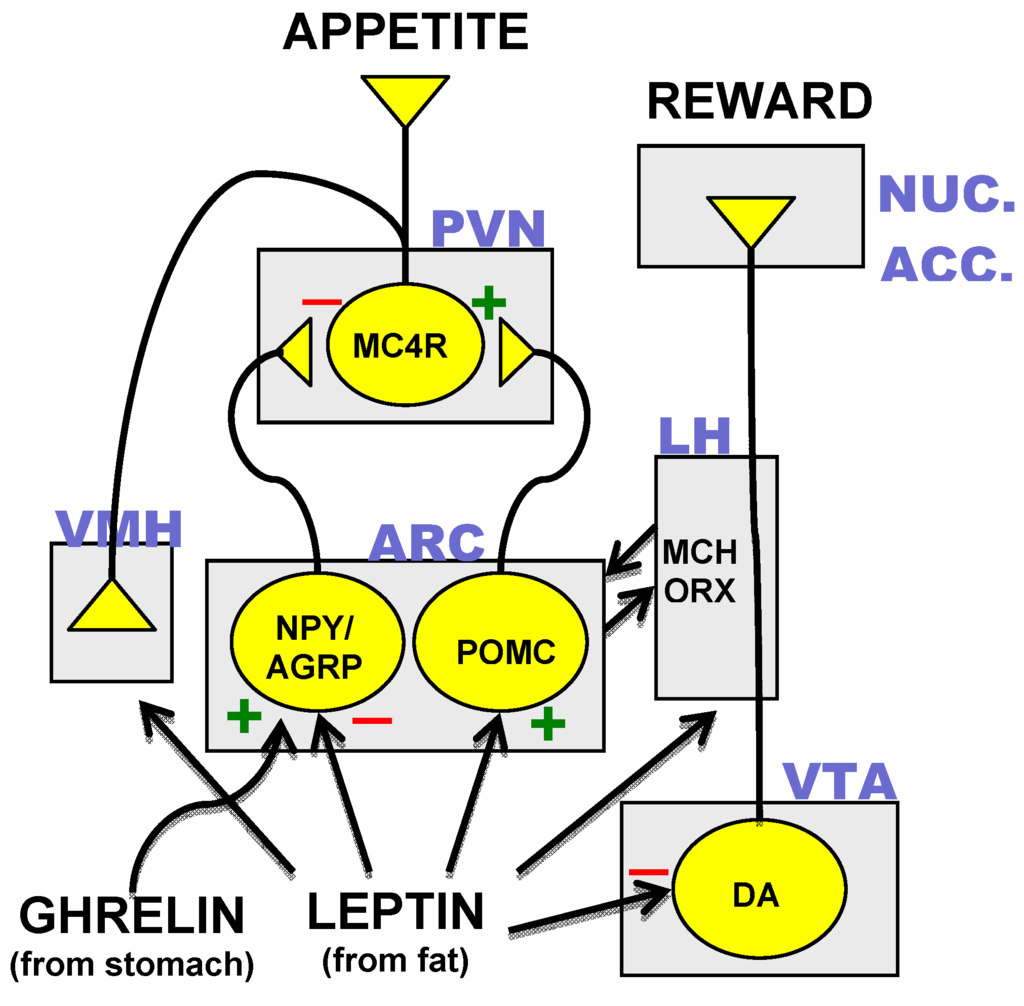
Healthcare Cost-free Full-text Pharmacological Support For The Treatment Of Obesity Present And Future The stomach-derived peptide hormonal agent ghrelin gets to the hypothalamus via the average prominence and stimulates homeostatic food intake through activation of NPY/AgRP neurons245, while boosting hedonic consuming through activation of dopaminergic nerve cells in the ventral tegmental area302. To activate its receptor, ghrelin calls for N-octanoylation (acylation) at its serine 3 residue, and as nutritional lipids are utilized for ghrelin acylation, this suggests that ghrelin could additionally serve as a nutrient sensor that educates the mind regarding incoming nutrients245. This section on future anti-obesity drugs focuses on tesofensine, considering that itis the only CNS acting anti-obesity medicine that has reached an innovative stage ofdevelopment. All other CNS acting medications remain in early in professional advancement andother than the minimal details on semaglutide and setmelanotide have actually nopublished tests for obesity therapy [112] Aminorex was approved for non-prescription sale as a treatment ofobesity in Austria, Switzerland and West Germany in 1965, but was never approvedin the United States [9]
Pharmacological Interaction With A Serotonin Cravings Suppressant
What is the most preferred anti weight problems medication?
Phentermine is the oldest and most extensively utilized weight management medication. It was originally made use of as a short-term medication to jump-start weight reduction, today more recent clinical guidelines have actually added it to long-term treatment. Some individuals might shed concerning 5% of their body weight by taking phentermine.
Activators Of Lipid And Energy Metabolism In Medication Growth
The resulting fat burning, particularly of new by mouth active GLP-1 agonists such as semaglutide is substantial, but is come with by stomach disturbances such as nausea or vomiting, vomiting, looseness of the bowels and dyspepsia which restricts maximization of the dose. To improve the metabolic results of GLP-1 agonists, mixes with other intestine hormonal agents such as GIP or glucagon to generate collaborating or corresponding actions have been checked out. Combination treatment produces tolerable signs but does not decrease stomach disturbances. On the other hand, sublingual therapy targeting the cell receptors for PYY on the tongue instead of the hypothalamic arcuate nucleus holds promise because the anatomic location of the Y2 receptors in the dental mucosa reduces the damaging systemic results of a centrally acting drug. Bupropion is a well-tolerated antidepressant that prevents reuptake of dopamine and norepinephrine and has been revealed to prevent hunger and food intake in numerous people.- Nonetheless, the enhancement in body weight was not statistically different relative to dose-titrated liraglutide.
- These altered organic mechanisms might discuss why short-term behavioural treatments are frequently inadequate for long-term weight-loss.
- Studies ofleptin deficient rodents and people demonstrated that the absence of the leptinhormone resulted in dark weight problems that was reversed by leptin hormone substitute, comparable to the condition of type-1 diabetes and its connection to loss of insulinsecretion [3]
- Mix therapy creates tolerable signs and symptoms however does not minimize intestinal disturbances.
- The European authorities removedsibutramine from the marketplace following the results of the precursor test.
Social Links