September 5, 2024
Tesofensine Peptide Evaluation: Benefits, Results, Dose, & Much More
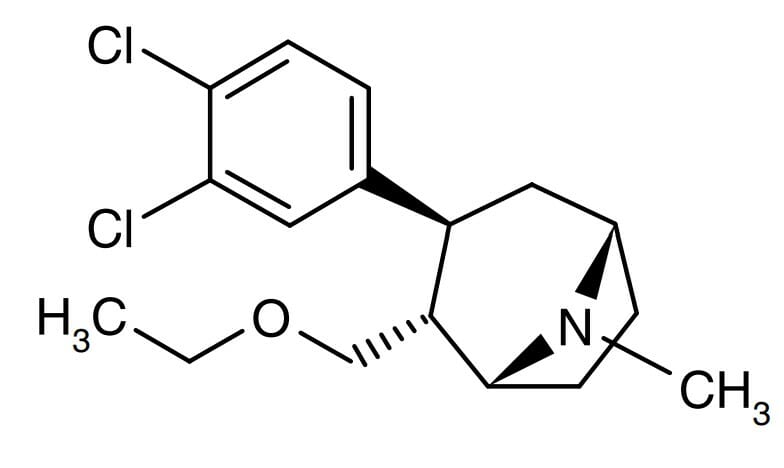
Saniona Discuss Article Addressing The Possible Mechanism Of Action Behind Tesofensine's Unique Weight Loss Impact The selective catecholaminergic mode of action of tesofensine separates it from the blended noradrenergic/serotonergic mechanism of sibutramine or the 5-HT2C receptor-mediated device of lorcaserin and d-fenfluramine. When tesofensine (1 or 2 mg/kg po) was provided to DIO rats for 28 days, it minimized the bodyweight of these animals by 5.7% and 9.9%, specifically (Hansen et al., 2010). Sibutramine (7.5 mg/kg po), which was the recommendation comparator in this experiment, created 7.6% weight-loss. If these results convert into scientific outcomes, tesofensine would have the potential to have equivalent or perhaps greater efficiency than sibutramine. Weight-loss caused by tesofensine in DIO rats was gone along with by improvements in metabolic standing that included decreases in abdominal and subcutaneous fat mass, reductions in plasma lipids and increased insulin level of sensitivity (Hansen et al., 2010). Complete statistical evaluations on body weight, food intake, and locomotor activity can be located in Supplementary Table 1. When rimonabant was withdrawn, all further development of taranabant was terminated (Aronne et al., 2010). In phase-II trials that included randomization to repaired doses of medication it was kept in mind that psychological side effects were the commonest factor for research attrition (Proietto et al., 2010). At the lowest dose there was enhanced vigor-activity; depression-dejection was seen on the highest possible dose. These evidently dopaminergic effects may result from harmony of the dopamine and endocannibinoid pathway (Despres et al., 2005). Although under activity of the benefit path can lead to discontentment and reduced state of mind, way too much stimulation can be habit forming and energizers are identified as medicines of abuse.Does tesofensine reason depression?
fat burning, and 32%of obese people had & #x 2265; 5%fat burning following 14 wk of treatment. Weight loss was come with by hypophagia, recommending a cravings suppressant action. Avoid Damaging Medication Occasions Today Tesofensine is a Serotonin-norepinephrine-dopamine-reuptake-inhibitor(SNDRI). SNDRIs are a class
of psychoactive antidepressants. Although losing 10 kg in 1 month is a big challenge and fairly difficult, you can still do it.

What Occurs When You Stop Cravings Suppressants?
A phase III trial will be completedin 2018 to examine modification in body weight in 372 grownups with excessive weight dealt with withplacebo, 0.25 mg or 0.5 mg tesofensine for 24 weeks. Tesofensine is a novel monoamine reuptake prevention that inhibits both norepinephrine, 5-HT, and dopamine (DA) reuptake feature. Tesofensine is presently in scientific advancement for the treatment of obesity, however, the medicinal basis for its strong impact in excessive weight administration is not clarified. Making use of a rat version of diet-induced weight problems (DIO), we identified the pharmacological systems underlying the cravings suppressive impact of tesofensine. DIO rats treated with tesofensine (2.0 https://devclouds.blob.core.windows.net/hiwenzba15kjas/sdkfjisdj/product-lifecycle/healthcare-totally-free-full-text-pharmacological-assistance-for-the-treatment.html mg/kg, s.c.) for 16 days revealed dramatically lower body weights than vehicle-treated DIO rats, being mirrored by a significant hypophagic action. Making use of an automatized food consumption monitoring system throughout a 12 h nighttime test period, tesofensine-induced hypophagia was checked out further by examining the severe interaction of a variety of monoamine receptor villains with tesofensine-induced hypophagia in the DIO rat.- Furthermore, there is proof that NE efflux enhances in the hypothalamus, including the PVN, throughout food usage (Stanley et alia, 1989; Morien et alia, 1995).
- The quantity of weight and fat cells that can be shed with tesofensine can vary among individuals, and it depends on a number of aspects including first body weight, general wellness, way of living behaviors, and adherence to a calorie-controlled diet and workout routines.
- This is believed to happen as an outcome of the body's anxiety action to the demand for sustenance.
- Weight-loss is an usual side-effect of the anti-convulsant medicine, zonisamide, and this prompted its evaluation as a treatment for weight problems (Gadde et al., 2003).
The Path Ahead For Weight Problems Medicines
The identification of this cell kind runs out the extent of this study, but it is appealing to hypothesize that most likely includes a large part of non-GABAergic neurons, maybe enriched of glutamatergic nerve cells. We acknowledge that our information can not dismiss the appealing opportunity that a different part of GABAergic neurons (from those prevented) can be triggered by tesofesnine. This is because activation of GABAergic neurons can cause oromotor stereotypy [13], comparable to that observed with phentermine and tesofensine at high concentrations (see listed below Fig 7). Refresher courses utilizing Cal-light or TRAP-like methods ought to be conducted to verify the identity of the activated neuronal sets recruited by tesofensine [48, 49]Contrast With Other Cravings Suppressants In Lean Rats
Karin Sandager Nielsen, CSO of Saniona, commented, "These data highly sustain the promotion of tesofensine as an unique reliable treatment for weight-loss in overweight people. The experiments demonstrate strong speculative abilities and scientific experience of the accountable scientists and their excellence in the area. The strategies made use of are highly sophisticated and well fit for demonstrating tesofensine's impacts at details neuronal pathway degrees and brings us closer to comprehending the mobile and network mechanism of action of tesofensine's special efficiency". Although tesofensine is largely utilized for weight loss, it has additionally been examined as a prospective treatment for several various other disorders such as major depressive disorder, Parkinson's condition, attention deficit disorder (ADHD) and Alzheimer's disease. Topiramate, a sulfamate derivative of fructose, is accepted for thetreatment of epilepsy and migraine headache treatment. In a dose acceleration test of 2 doses each day, the topiramatedose was increased biweekly by 16 mg to doses of 64, 96, 192, and 384 mg/d andthe resulting weight-loss were 5%, 4.8%, 6.3%, and 6.3%, respectively with theplacebo group losing 2.6%.Social Links